Abstract
Introduction:
Thrombophilias augment the risk of pregnancy related morbidity, increasing the likelihood of adverse events such as fetal loss [1,2]. Current guidelines recommend women be tested for antiphospholipid antibodies when they present with recurrent miscarriage (3 or more consecutive pregnancy losses before 24 weeks) or with a late miscarriage (loss between 12-24 weeks) [1]. Inherited thrombophilia testing (including factor V Leiden, prothrombin gene mutation and protein S) is also recommended for women with 2nd trimester miscarriage [1]. Controversy exists around the benefit of universal thrombophilia screening given the low relative increase in risk of pregnancy events and the lack of evidence surrounding interventions such as low molecular weight heparin (LMWH) and aspirin in most thrombophilias [2,3].
Our aim was to evaluate our own testing for thrombophilia in pregnancy related events. We aimed to assess the frequency of positive results and evaluate whether testing resulted in a change in management for our cohort of women.
Methods:
Data was collected retrospectively on 151 thrombophilia screens requested for obstetric reasons from July 2016 to July 2017 at Waikato Hospital. The tests comprised antiphospholipid antibodies (APL) including lupus anticoagulant (LA), factor V Leiden (FVL), protein C and S levels, antithrombin III (ATIII) and prothrombin (PT) gene mutations. Data pertaining to request information, outcome of current and previous results were included. Cost analysis was done using NZ $300 (US $223) for a full thrombophilia screen and NZ $30 (US $22) for protein S alone. GraphPad Prism 7 software was used for all data interpretation.
Results:
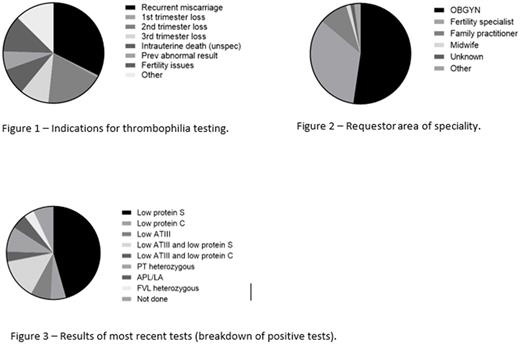
The mean patient age was 31 years (range 18-48 years). The commonest reason for referral for testing was recurrent miscarriage (49/151) (Figure 1) and the majority of requests were from the obstetrics department (79/151) (Figure 2).
83% (126/151) of tests requested were for a full thrombophilia screen; the remainder (25/151) were for single tests or a combination. The majority were being tested for the first time; 16.5% (25/151) had previous testing done. Of those with previous tests 52% (13/25) had no abnormality found; the others had low protein S levels or ATIII deficiency, or both.
With the current test, almost two thirds of patients (62%, 94/151) had no abnormality found. Of the others, low protein S levels made up the majority of positive tests (26/57) (Figure 3). Of these 4/26 tests were repeated with 1 ongoing low result representing a likely true protein S deficiency. The laboratory cost of negative tests was estimated to be NZ $28,200 (US $20,992); while the cost of testing for protein S, including repeat testing due to low levels likely associated with pregnancy, was estimated at NZ $879 (US $648).
Results that could potentially change management (APL antibodies, presence of VTE with positive inherited thrombophilia result) were few (6/151).
Conclusion:
In our tertiary centre, the majority of thrombophilia testing done for obstetric reasons over a 12 month period was negative. Most positive results (low protein S) were likely attributable to recent pregnancy, with a lack of repeat testing undertaken. There was a potential change in management for only 6 patients. Our results are in line with the literature which shows a scarcity of evidence supporting thrombophilia screens in pregnancy related morbidity. These tests are not only costly but for the majority provide no clinical benefit. Our next step is to disseminate these results locally and we aim to educate our colleagues on the utility of thrombophilia testing in pregnancy related events and when it is clinically helpful. We await with interest the results of ongoing randomised controlled trials assessing the role of LMWH and aspirin in thrombophilia in pregnancy.
[1] Royal College of Obstetricians and Gynaecologists (RCOG) Green-top guideline No 7. The investigation and treatment of couples with recurrent first-trimester and second-trimester miscarriage. April 2011.
[2] Baglin T, Gray E, Graves M, et al . Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149(2): 209-20
[3] de Jong PG, Kaandorp S, Di NM, et al . Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev . 2014;7:CD004734
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal